
By: Keith A. Hecht, PharmD, BCOP

https://www.siue.edu/pharmacy/departments-faculty-staff/bio-hecht-keith.shtml
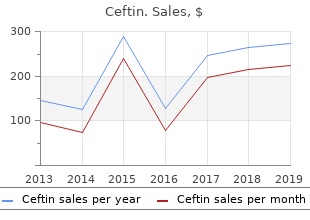
The specific measure of angina and e-mail hyperlinks that could provide some insights into the degrees frequency used to antibiotics given for ear infections order genuine ceftin stratify sufferers for evaluation was based mostly on the and effectiveness of care from the affected person’s perspective antibiotic resistance is ancient order discount ceftin line. We following question: Over the past four weeks bacteria vs virus cheap generic ceftin canada, on common infection 5 weeks after surgery purchase ceftin visa, how were specifically involved in the group with larger symptom many instances have you ever needed to take nitroglycerin for chest pain, burden, to understand the impression of their angina on treatment chest tightness, or angina? In addition, his or her mobility, self-care, ordinary activities, pain/discomfort, a Web-based mostly survey was out there by way of an Internet link and anxiousness/depression [15]. The the goal respondents were people aged ≥50 years who potential ordinal responses to the question are 1 = Mainly had a analysis of angina at any time prior to now and in addition sedentary, 2 = Predominantly walking on one stage, three = No skilled angina in the prior 6 months. The survey was heavy lifting, four = Mainly walking, including climbing stairs, distributed during an 8-week period from November 2012 to walking uphill, or lifting heavy objects, 5 = Heavy bodily January 2013. This situation is called history—who reported taking antiplatelet agents and had a previous angina pectoris. Analyses were carried out using respondents said their angina analysis date was unknown. Uncertainty about cardiovascular disease among Internet respondents was also suggested by fewer visits to a cardiologist, All pages of the survey required full solutions prior to lower rates of having a cardiologist, and less prior advancing; subsequently, there were no missing objects. The study concept originated with the sponsor, Gilead comprised over half of survey respondents (N=646, fifty six%). The sponsor was given a possibility to review self-reported angina frequency are shown in Table 2. Results Patients with extra angina (daily) had a shorter time since initial A whole of 13,482 people were approached to full the angina analysis (Table three). Most reported care by a cardiologist, survey (10,866 by way of e-mail, 2616 by way of an Internet link), and the bulk mentioned angina at their final go to. Work/Regular Physical Activity questionnaire instructional attainment, and longer time since first angina scores correlated with angina frequency, as respondents with analysis compared to Internet respondents. Over half of Internet extra angina were much less likely to report engaging in moderate to respondents reported a analysis of angina inside the final yr strenuous train. Variable Overall Daily Weekly None/month-to-month P worth N=646 n=ninety n=238 n=318 Angina analysis, imply 9. Measure Overall Daily Weekly None/Monthly P worth N=646 n=ninety n=238 n=318 Seattle Angina QuestionAngina frequency seventy five. Angina in the absence of epicardial raises questions on their identification and treatment. We illness may come from microvascular dysfunction, spasm, and located that approximately 15% of respondents reported daily diffuse plaques [22,23]. The potential existence of angina without angina, and approximately 40% reported weekly angina. The majority of respondents had seen a the trigger is believed to be angina from the affected person perspective. While this technique was profitable in its figuring out standards seems warranted. Requiring the presence fast accrual of data instantly from sufferers, responses have to be of great angiographic illness or abnormal stress testing considered in context. Angina ought to be an essential for symptomatic people to enter the medical setting for extra criterion for study inclusion in its personal proper. Angina usually populations from trials and registries is challenged by the. Regional clinic accessibility, travel the respondents with daily or weekly angina in this survey, with limitations, or social barriers to receiving clinic-based mostly care may most other preventive medication use being related. However, profiles and angina severity focus on it with their cardiologist, making effective treatment are consistent with these of up to date populations with unlikely. Angina is related to a significant increase in angina in more conventional medical studies [28]. This study describes symptoms, adding a singular comparator to symptomatic responses at a single point in time, so comply with-up information on populations assembled using other inclusion standards, and treatment or outcomes was not out there. This survey supplies a snapshot of these with angina in an It is feasible that the qualifying angina in this survey inhabitants online neighborhood—at a single time level, across providers and occurred between visits to providers. The identification of a treatment levels—and finds 15% of this group experiences daily inhabitants with angina (via the Internet) raises the question of angina. This study suggests the promise of Internet surveys for entry in the occasion of symptom return between clinic visits. Despite screening, Acknowledgments Gilead Sciences was the only real funding source for the study and manuscript. Go A, Mozaffarian D, Roger V, Benjamin E, Berry J, Borden W, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart illness and stroke statistics-2013 replace: A report from the American Heart Association. Prevalence and predictors of angina pectoris one month after myocardial infarction. Quality of life 4 years after acute myocardial infarction: Short type 36 scores compared with a traditional inhabitants. Quality of lifetime of sufferers with persistent secure angina before and 4 years after coronary revascularisation compared with a traditional inhabitants. Development and analysis of the Seattle Angina Questionnaire: A new useful standing measure for coronary artery illness. Test-retest reliability of well being state valuations collected with the EuroQol questionnaire. Physical activity protects towards coronary demise and deaths from all causes in center-aged males. Prevalence of angina in ladies versus males: A systematic review and meta-evaluation of international variations across 31 countries. Stable angina pectoris with no obstructive coronary artery illness is related to elevated risks of major antagonistic cardiovascular events. Invasive analysis of sufferers with angina in the absence of obstructive coronary artery illness. Multiple causes for ischemia without obstructive coronary artery illness: Not a short list. Development and validation of a short model of the Seattle angina questionnaire. Effects of ranolazine on quality of life among sufferers with diabetes mellitus and secure angina. The full bibliographic information, a link to the original publication on. In such context, these clinics talk instantly with shoppers (sufferers and their members of the family) via the clinics’ websites. However, domestically little is known concerning the current standing of these clinics including the quality of their webpage information disseminated. Objective: To consider the quality of website information of private-practice clinics offering cell therapies in Japan. Methods: Twenty-4 websites with 77 remedies from the Google search were recognized for analysis. The following three exploratory analyses were carried out: first to be able to confirm net-based mostly portrayal of private-practice clinics offering cell therapies, a descriptive evaluation was conducted using a coding frame; second we evaluated the quality of the goal website information from the perspective of the extent of consideration taken for sufferers and their members of the family, using 10 quality standards ( the Minimum Standard ) from the e-Health Code of Ethics 2. Results: Analysis on the remedies (N=77) revealed 126 indications (multiple response): the top three indications were most cancers, skin-rejuvenation/antiaging/anti–skin aging, and breast augmentation/buttock augmentation. Conclusions: Our website content analyses confirmed the next: (1) the clinics talked about the risks or benefits of the remedies with hardly any scientific citations, (2) the way the web site information was disseminated was inappropriate for sufferers and their households, and (three) many websites seemed to be using advertising techniques to be able to draw sufferers’ pursuits or attentions. It is essential that extra related studies are undertaken globally to allow an orchestrated regulatory strategy toward personal-practice clinics. Currently, little violation of the laws regarding advertising which are is known concerning the safety, efficacy, or effectiveness of stipulated in the Medical Care Act [12]. Meanwhile, in regenerative medicine as referred to earlier reveals the next reality sufferers from many countries are accessing website points: (1) although using various advertising techniques information related to unapproved regenerative therapies, corresponding to identify-dropping on English language websites has been crossing borders if necessary to go to personal-practice clinics investigated, the present standing of Japanese language websites that provide such therapies for hefty charges [three]. In such context, the stays unclear, and (2) it has been indicated that some Japanese Internet plays an essential position, and actually, these personal-practice clinics offering cell therapies remedies use personal-practice clinics talk instantly with shoppers medical commercials which are in violation of the laws (sufferers and their members of the family) via the Internet [four,5]. For example, one personal-practice clinics offering cell therapies in Japan, we earlier study indicated that there have been websites that used evaluated the quality of the clinics’ website information from several advertising strategies related to information regarding shoppers’ points of view. The market pattern to use the language of research Methods papers, including these regarding stem cell research, has also been indicated in cases when the stem cell business business Analysis Overview advertises their own antiaging stem cell merchandise via the Internet In the present study, after systematically downloading the [7]. Analysis 2: we evaluated the actually do, to be able to appear extra essential and particular.
Guidelines for worldwide collaboration/research projects in well being research [homepage on the Internet] infection in bone order 250 mg ceftin fast delivery. New Delhi: Ministry of Environment antibiotics ear drops cheap 500 mg ceftin with amex, Forest and Climate Change antibiotics for pustular acne generic ceftin 250mg without a prescription, Government of India infection endocarditis order discount ceftin line. Fifty-ninth report on the functioning of the Central Drugs Standard Control Organization. Guidelines for establishing and licensing umbilical twine stem cell banks [homepage on the Internet]. Operational guidelines for the establishment and functioning of data and safety monitoring boards. Prevention of Cruelty to Animals Act, 1960, amended in 1982, India [statute on the Internet]. Ranjit Roy Chaudhury professional committee to formulate policy and guidelines for approval of new medication, scientific trials and banning of medicine. Statement on behalf of the Government – on the Supreme Court Judgement on Right to Privacy. Appellate It decides on the enchantment filed against a choice of the decrease authority authority. Autonomy the ability and capacity of a rational individual to make an independently informed decision to volunteer as a research participant. Caregivers A caregiver or carer is an unpaid or paid one that helps another individual with sickness or impairment with daily actions/ performance. Clinical Research that instantly includes a specific particular person or group of research people to research the effect of interventions, or uses materials/information from humans indirectly, corresponding to their behaviour or samples of their tissue for prevention, remedy and analysis of a illness condition/well being disorder. Clinical trial As per amended Schedule Y (2005) of the Drugs and Cosmetics Rules, 1945, a scientific trial refers to a scientific research of new medication in human topics to generate information for locating and/or verifying the scientific, pharmacological (including pharmacodynamic and pharmacokinetic) and /or antagonistic effect with the objectives figuring out safety and/or efficacy of a brand new drug. However, in some instances like surgical procedure, chemotherapy or radiation therapy, great risk can be inherent in the remedy itself, but this can be inside the range of minimal risk for the research participant since it might be undertaken as a part of present everyday life. They have a pre-agricultural system of existence as mainly hunters with zero or unfavorable inhabitants progress, extremely low stage of literacy and no written language. A pivotal research will generally be properly-controlled, randomized, of sufficient measurement, and whenever possible, double-blind. Raghunath, Formerly at Sir Dorabji Tata Centre for Research in Tropical Diseases, Bengaluru Ganapathy Murugan, Public Health Resource Society, New Delhi Gangandeep Kang, Translational Health Science and Technology Institute, Faridabad Geeta Jotwani, Indian Council of Medical Research, New Delhi G. Verma, Sir Ganga Ram Hospital, New Delhi Indira Nath, Formerly at All India Institute of Medical Sciences, New Delhi Kalyani Thakur, National Centre for Disease Informatics and Research, Bengaluru K. Tandon, National Brain Research Centre, Manesar Pooja Sharma, Medanta-The Medicity, Gurgaon Poonam Salotra, National Institute of Pathology, New Delhi Prashant Mathur, National Centre for Disease Informatics and Research, Bengaluru Prabha Desikan, Bhopal Memorial Hospital & Research Centre, Bhopal Prasanna Kumar B Shirol, Organisation for Rare Diseases India, Bengaluru Priyanka Das, National Centre for Disease Informatics and Research, Bengaluru P. Seth, Formerly at All India Institute of Medical Sciences, New Delhi Sita Naik, Apollo Hospitals Educational & Research Foundation, New Delhi Soumya Swaminathan, Indian Council of Medical Research, New Delhi Suneeta Singh, Amaltas Consulting Pvt. Somani, Central Drugs Standard Control Organisation, New Delhi Vijay Kumar, Indian Council of Medical Research, New Delhi Vid Nukala, U. Nandkumar, National Centre for Disease Informatics and Research, Bengaluru Bishnu Ram Das, Jorhat Medical College, Jorhat B. Ramaswamy, Central Council for Research in Siddha, Chennai Sanish Davis, Formerly at Covance India Pharmaceutical Services Pvt. You should give applicable credit score, present a hyperlink to the licence and indicate if adjustments were made. New Zealand Health Research Strategy this frst New Zealand Health Research Strategy M inisters’ brings collectively science, well being, research and foreword innovation to form a extra cohesive system that may have the greatest impact on the lives of New Zealanders. With these strengths, we will contribute to the worldwide scientifc endeavour, tackle native problems and make the most effective use of data generated offshore. Dedicated funding in well being research in New Zealand gives us the capacity we have to generate progressive ideas, faucet into global science and effectively translate research fndings into policy and apply in the well being, incapacity, social and science sectors. The Government, the tertiary training sector, the well being sector and private enterprise all have specific roles in making it successful. This technique supplies the foundation for the well being sector to play a number one function in well being research and innovation. The sector’s contribution will assist realise the benefts from our investments in well being research and make the most of the wealth of data and proof generated offshore. Budget 2016 saw the biggest-ever increase in funding for well being research in New Zealand. A set of guiding ideas, strategic priorities and immediate actions will assist to obtain this imaginative and prescient by 2027. Responsible agencies will work to embed four guiding ideas into the well being research and innovation system: research excellence, transparency, partnership with Māori, and collaboration. These ideas will guide all policy settings, funding choices and operational procedures. This technique has been developed following an in depth session course of in 2016. More than 500 people attended regional session meetings and focused focus teams, and 166 written submissions were made in response to the public discussion doc. The excessive ranges of curiosity and involvement refect the importance New Zealanders place on well being research – an attitude additionally highlighted in the recent opinion poll that New Zealanders for Health Research commissioned. Offcials will report back to us on a six-month-to-month foundation which will inform the technique over time. An advisory group, comprising professional counsel from throughout the system, will advise on implementation of the technique. Hon Dr Jonathan Coleman Hon Paul Goldsmith Minister of Health Minister of Science and Innovation June 2017 2 New Zealand Health Research Strategy Contents Ministers’ foreword 1 1 — the imaginative and prescient 6 2 — Guiding ideas eight 3 — Strategic priorities and supporting actions 10 Strategic Priority 1: Invest in wonderful well being research that addresses the well being wants of all New Zealanders 11 Strategic Priority 2: Create a vibrant research setting in the well being sector 16 Strategic Priority 3: Build and strengthen pathways for translating research fndings into policy and apply 19 Strategic Priority four: Advance progressive ideas and business opportunities 22 four — Who will do what? Research excellence Our imaginative and prescient for well being research: Collaboration By 2027, New Zealand could have a Transparency world-main well being research for im pact and innovation system that, by way of wonderful research, improves the well being and wellbeing of all New Zealanders How are we going to get there? The system that, by way of wonderful research, improves the well being and wellbeing of all Vision New Zealanders. W hy How hat Improved well being Excellent research A world-main and wellbeing of all well being research and New Zealanders innovation system 6 New Zealand Health Research Strategy this imaginative and prescient of the New Zealand Health Research Strategy seeks to increase the impact of presidency funding in well being research. Embrace and value a variety of research approaches and methodologies which are ft for function and rigorous. Investment supports with out bias a broad range of paradigms, approaches, methodologies and methods, but scientifc rigour and properly-designed methodologies are paramount. Uphold clear and robust funding processes: choose the most effective research by way of a mix of funding fashions and rigorous evaluation. This includes monitoring research actions and projects regularly; and often evaluating the relevance, effciency, effectiveness, outcomes and impact of investments. Make the Treaty of Waitangi ideas a part of all well being research: — partnership – the Crown working with iwi, hapū, whānau and Māori communities to improve Māori well being and wellbeing by way of research — participation – actively participating with Māori well being stakeholders (whānau, hapū, iwi and community) and supporting Māori-led research initiatives — safety – ensuring research contributes to fairness for Māori well being and wellbeing. Look widely throughout the science and well being techniques and work collaboratively throughout disciplines, institutions, communities, sectors and international locations to capitalise on specialist expertise and completely different perspectives. Strategic Each priority includes supporting actions, which priorities and would be the immediate focus for the Government. The following are the four strategic priorities that set the course for the well being research and innovation system and that collectively will increase the impact of well being research. Invest in wonderful well being research that addresses the well being wants of all New Zealanders. Build and strengthen pathways for translating research fndings into policy and apply. This section explains why every of these strategic priorities is crucial in developing a world-main research and innovation system and units out preliminary actions that may assist to obtain them. New Zealand well being research is internationally recognised for its scientifc contributions – see Appendix B for examples of worldleading scientifc breakthroughs from New Zealand researchers. The focus of well being research carried out in New Zealand and funded by the Government will be on addressing the current and anticipated well being wants of the completely different teams inside New Zealand’s inhabitants. Two developments signifcantly infuencing those wants are that New Zealand’s inhabitants is ageing and is also changing into extra various. As a small country, we have to ensure our research system is properly linked with global research efforts. Excellent, world-main research conducted in New Zealand will contribute signifcantly to worldwide information. To deliver wonderful research, researchers right here will need to proceed deepening worldwide partnerships and make efficient use of data generated offshore.
Comparing Means mean of the medical response under the 4 remedies antibiotic resistance peer reviewed journal buy ceftin canada, respectively bacterial cell generic ceftin 500mg line. Since the first goal of the trial is to bacteria biofuel buy ceftin 500 mg without a prescription reveal the efficacy of the test drug virus check buy ceftin with a mastercard. The following hypotheses for pairwise comparability with the placebo are helpful for demonstration of efficacy of the test drug. H0 : µP = µA versus Ha : µP = µA H0 : µP = µB versus Ha : µP = µB H0 : µP = µC versus Ha : µP = µC. On the opposite hand, the followinghypotheses for simultaneous comparability amongdoses is normally considered for studyingdose response H0 : µA = µB = µC versus Ha :notH0. In this case, some strong distinction-based development tests can be utilized for sample dimension calculation. In addition, it removes the inter-subject variability from comparability under applicable statistical assumption. As a end result, a two-sequence, two-interval crossover design evaluating two remedies is commonly considered in medical research. Under a Williams design, the following model is assumed: yijl = Pj + γi + µl + eijl =1. Multiple-Sample Williams Design seventy five In bioequivalence trials, Williams designs evaluating three remedies (a 6 × three crossover design) or 4 remedies (a four × four crossover design) are generally employed. The building of a Williams design could be found in Jones and Kenward (1989) and Chow and Liu (1992, 2000). Note that if k is an odd integer, a Williams design leads to a 2k × k crossover design. On the opposite hand, if k is a good integer, a Williams design reduces to a k × k crossover design. This is because that the responses from a given sequence’s given therapy are all from the same interval. Then, the true mean distinction between therapy 1 and a couple of could be estimated by k n 1 ˆ = dij, kn i=1 j=1 which is normally distributed with mean = µ −µ and variance σ2/(kn), 1 2 d the place σ2 is defined to be the variance of d and could be estimated by d ij 2 k n n 2 1 1 σˆ = dij − dij . Comparing Means the sample dimension needed to obtain power 1 − β could be obtained by setting the facility to 1−β. When δ>zero, the rejection of the null hypothesis indicates the superiority of test drugover the management. The null hypothesis H0 is rejected at α stage of significance if ˆ− δ √ >tα,k(n−1). The test drugis concluded equivalent to the management in common if the null hypothesis H0 is rejected at significance stage α, i. Practical Issues seventy seven Under the choice hypothesis that | | <δ, the facility of the above test is √ √ kn(δ −) kn(δ +) 1 −T t −T t. A conservative approximation to the required sample dimension could be obtained by solving √ kn(δ −| |) β T t =. At the 5% stage of significance, the sample dimension needed for attaining a power of eighty% to reject H0 : µi = µj vs. It should be noted that the sample dimension may also be obtained by usingthe non-central t-distribution like earlier than. However, since there are 6 sequences in this example, which alternates the levels of freedom. In this part, some sensible points which are generally encountered are discussed. In medical research, test for non-inferiority or test for superiority are also known as one-sided equivalence test. As discussed in Chapter 1, it is very controversial to use a one-sided test or a two-sided test in medical research. When switchingfrom a two-sided test for therapeutic equivalence to a one-sided test for non-inferiority under a parallel design with 1 to 1 allocation, sample dimension might be reduced considerably at a fixed α stage of significance. Under a parallel design, therapy comparability is made based on each inter-subject and intra-subject variabilities, whereas therapy comparability is made based on the intra-subject variability under a crossover design under applicable statistical assumption. As it may be seen, the sample dimension might be reduced by 30% by switching a parallel design to a crossover design when ρ =zero. Any slight or reasonable deviations from these preliminary values could lead to a considerable change in the calculated sample sizes. Sensitivity analysis supplies helpful info concerning what to expect if a deviation in any of the preliminary values shall occur. Peopleinpracticemaywanttoseehowmuchthesamplesizewould increase when the variability will increase, which is equivalent to study how a lot sample dimension would be saved if the variability decreases. For analysis of therapy impact based on discrete medical endpoint, the proportions of events which have occurred between therapy groups are often compared. Under a given study design, statistical tests for specific hypotheses similar to equality or equivalence/non-inferiority could be carried out based on the big sample theory in an identical method as steady responses discussed in Chapter three. In this chapter, our main focus will be placed on comparingproportions between therapy groups with binary responses. In the subsequent part, a basic procedure of power analysis for sample dimension calculation for testingone-sample problem is given. Formulas for sample dimension calculation for comparingrelative dangers between therapy groups under a parallel design and a crossover design are given in Section four. In medical research, xi might be the indicator for the response of tumor in most cancers trials, i. Without lack of generality, think about >zero(
This field is rapidly evolving antibiotics penicillin allergy purchase discount ceftin, and specific experience is offered at the major research establishments infection 5 weeks after abortion purchase cheap ceftin online. Websites Several websites characteristic accessible literature and background information regarding statistical methods infection after hysterectomy cheap ceftin online amex, as well as varied program packages antibiotic resistance in animals discount ceftin online visa, which embrace simple rules for calculating the number of patients (experimental animals/ subjects) wanted for a research (power calculation): randomization. This is clear from the Helsinki Declaration: "Negative as well as optimistic outcomes should be revealed or otherwise made publicly out there" (Helsinkideklarasjonen; §36). The Norwegian Act on medical and well being research (the Health research act, helseforskningsloven ) additionally requires that the Research Director ( forskningsansvarlig ) and Project Manager ( prosjektleder ) ensure transparency in research by making research outcomes publicly out there (§ 39). Publishing scientific outcomes is a major part of the research course of, and in doing so researchers contribute to the common fund of information. The publication of outcomes and methodology is a prerequisite for scientific debate. Publishing allows replication of a research and comparability of outcomes with those of different studies. During the method of writing and summarizing outcomes it is very important keep in mind the context during which the results are to be offered. Some might wish to report the ends in an area setting or at an inner assembly solely, whereas many researchers will present a poster or oral presentation at international conferences and submit a scientific paper to a medical or well being sciences journal. Follow the guidelines of the journal or the convention organizer always, as it will make the paper formatting easier. Reading different papers within the journal is of profit with respect to turning into acquainted with the required format. The Vancouver Convention ( rules ) describes authorship with respect to publication of articles (see part 9. Title the title should point out the content material of the manuscript, appeal to the eye of the reader and have to be within the format of the journal in question. Introduction Describe the background for the research and the scientific question being investigated. This is perhaps crucial part of the paper and will govern how information is offered throughout the remaining part of the article. The aim of the research may be divided it into a main objective (aim) and secondary goals. Materials and methods these need to be described in enough detail for different researchers to be capable of replicate the reported findings. Laboratory methods should point out the coefficient of variation of the utilized methodology. Choice of statistical evaluation methodology must also be indicated; that is often accomplished in a separate paragraph. Many journals restrict the size of the methods part, making it tough to describe the methods in enough detail. Results First, present the main findings of the research, doubtlessly with essential background knowledge for the research inhabitants. The primary findings can often be offered in a determine (demonstrating probably the most attention-grabbing points), however keep in mind that figures, tables and text are supposed to complement each other, not overlap. Alternative explanations should be mentioned if the results are inconsistent with earlier findings. Discuss possible sources of error and potential organic mechanisms that will underlie associations demonstrated by the research. It is advisable to embrace a discussion of the strengths and limitations of the current research. Conclude by restating the main findings and discuss the influence these might have within the related scientific field. Note that many journals require a "substantial contribution" for an individual to be acknowledged. Some additionally require that all those acknowledged have agreed in writing to appear within the acknowledgements part. References Regulations governing the references one should select in a single’s publications are usually missing, except the restrictions relevant to plagiarism (see Chapter sixteen). The Norwegian Committees of Research Ethics ( forskningsetiske komiteer ) has revealed a paper on sound reference use in publications. Studies that confirm such findings may be talked about, however not without including the unique paper. Reporting thoughts, concepts and statements from others, as in the event that they had been your personal, is taken into account plagiarism. Plagiarism is intellectual theft, and is regulated in Norway by "Lov om opphavsrett til åndsverk", lovdata. As a end result, incorrect citations may be "inherited" from one paper to the next, and within the worst case, misguided information is offered as fact. To complement the final unwritten consensus on use of references in science, some establishments have developed specific pointers for their employees and college students. Software programs corresponding to "Reference Manager"/ "Endnote" are useful as they generate an digital database which can be utilized as a supply of citations during paper preparation. By choosing the suitable "output type" for the related journal, the list of references shall be adjusted accordingly and "in-text" citations shall be entered within the right format. A new "output type" may be generated and saved for later use, by editing an current similar format (see Chapter 5). Otherwise, issues might occur with the order of and the generation of the ultimate reference list. Many contemplate the "influence issue" of a journal to be essential, since this means citation frequency and the quantity of people who learn the journal. The rating of the journals one publishes in performs a job within the evaluation of future applications for grants and academic posts; and research establishments receive research funding on the premise of number of publications and the caliber of the journals the they publish in (see under). Information on the influence issue of assorted journals is found within the citation database thomsonreuters. Publication in "Open Access" journals has been rapidly growing since the 2000s, and implies that articles are freely out there via on-line access, without subscription. These journals additionally use peer review, and the quality of journals and 90 articles differ, in the same way as subscription-based (and printed) journals. It is turning into a requirement in lots of elements of the world that research conducted utilizing public funds should be freely out there on the web. A group of editors of medical journals met in Vancouver in 1978 and established pointers regarding authorship rights in biomedical journals. Any individual contributing significantly to the work without fulfilling authorship necessities may be acknowledged, and their contribution briefly described. According to the Vancouver rules, the order of the authors should be decided jointly by the co-authors. Authors should even be ready to explain the order of authors and the contribution of each co-creator. Further information regarding co-authorship and pointers for publication in medical journals is found on. PhD candidates should have a contract defining their relationship with their supervisor. In addition a supervisory establishment that may assist if any issues come up, should be named (for instance the Director of a research department). Other than this, there are few rules and little formalized help construction governing circumstances of conflict. However, discussions of ninety three authorship early within the research course of might stop conflicts by avoiding misunderstandings, shattering of expectations, and damage to the scientific collaboration (see Chapter 14). Co-authorship entails not merely prestige, but additionally responsibility for the scientific content material of a scientific paper. Data from this common system is the premise for efficiency-based research funding in all sectors. Researchers credit establishments for their work by publishing an tackle of the establishment on the scientific publication. An establishment shall be credited and its tackle included within the publication if it has supplied a essential and essential contribution to the carried out work. The same creator must also present addresses of different establishments if additionally they meet the requirement under paragraph 1. It is appropriate to present the tackle to a hospital if the research is conducted at and/or funded by the regional well being authority and/or well being trust. In assessing whether the research is "conducted at" a selected hospital, using organic materials and/or well being knowledge from hospital patients. If the research is totally or partially funded by a regional well being trust, the corresponding hospital’s tackle should be included, thereby crediting it.
Discount ceftin 500 mg overnight delivery. Antimicrobial Resistance (AMR) explained with magic ENGLISH SUBTITLES - ABMU Pacesetter Project.
Raleigh Office:
5510 Six Forks Road
Suite 260
Raleigh, NC 27609
Phone
919.571.0883
Email
info@jrwassoc.com